CoProjects
Collaborate with CoLabs
Research projects are becoming increasingly complex, requiring expertise in multiple disciplines, specialized equipment, and integration of multi-modal data. CoLabs has established a CoProject model where we collaborate with you to remove these barriers.
CoProjects are cross disciplinary studies that drive collaborations between multiple CoLabs teams and the principal investigator’s team. The CoProject model highlights our collaborative culture, and we work with both UCSF and external investigators. CoProjects are also conduits for CoLabs to develop new methodologies and technologies that can be shared with the scientific community. You can learn more about our active and completed CoProjects in our Portfolio.
If you have further questions or are interested in initiating a CoProject, please reach out to CoLabs to arrange an informational meeting.
Getting Started
Workflow
Both collaborators and CoLabs investigators are involved in all stages of each CoProject, from grant preparation to data generation and analyses to publications. Where appropriate and feasible, collaborators are physically embedded in CoLabs where they will have access to experts and facilities needed for the work.
CoLabs also works closely with the Office of Collaborative Research. They provide additional assistance with grant writing, clinical coordination, and project management.
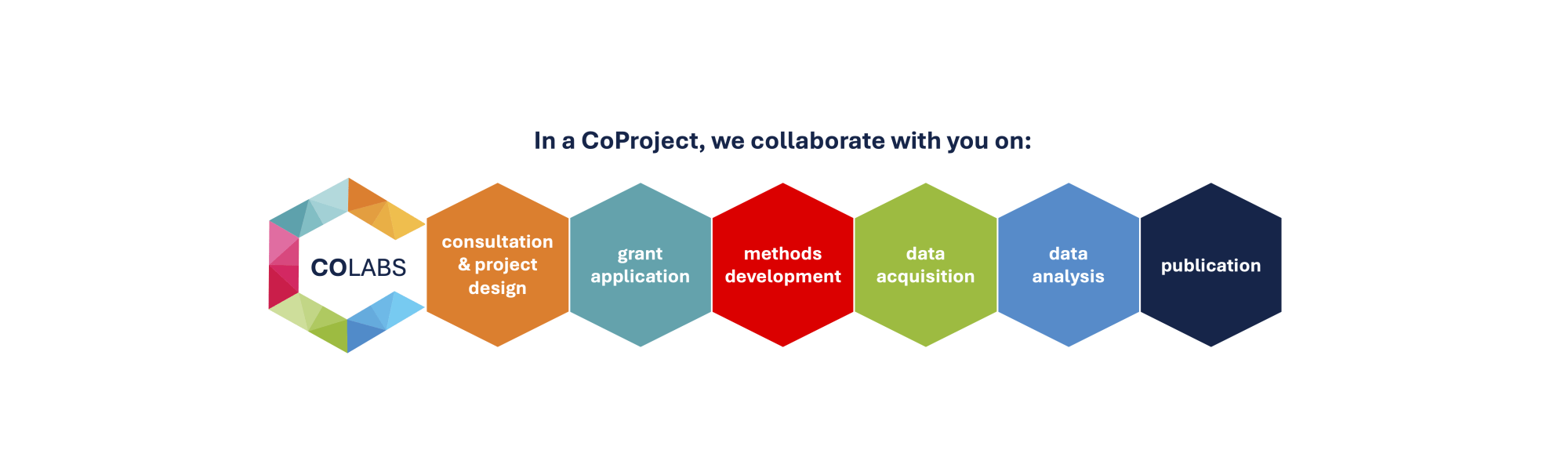
Initiate a CoProject
When you are ready to initiate a CoProject, please reach out to us for an informational meeting. We will then follow-up with an introductory meeting with the relevant CoLabs teams to discuss your study in more detail.
Obtaining Funds
We offer assistance with grant preparation to our CoProject collaborators and provide guidance with CoLabs-related budgets, facilities and resources, and letters of support.
CoLabs researcher salary support and other costs related to work to be done in CoLabs are funded through grants and contracts from NIH and other agencies and from industry partners or through philanthropic sources. We work with investigators and their teams to prepare applications for individual research projects or for multicomponent research programs submitted in response to many different funding mechanisms. The UCSF ImmunoX Initiative is a major supporter of CoLabs and has annual CoProject RFAs. CoLabs often partners with the UCSF Office of Collaborative Research, which offers additional resources relating to grant writing and project management.
We also support core resources connected with Center and Program Project grants. These can be powerful tools for advancing shared resources at UCSF.
Please let us know as soon as possible if you are considering submitting an application so we have sufficient time to help you put forward a strong proposal.
How CoProject collaborations work
The collaborating PI will select a member of their group or hire a scientist to be the dedicated embedded researcher for the CoProject. Embedded researchers are the collaborators who drive the CoProject day-to-day. When appropriate, they are physically based in CoLabs where they will have access to experts and facilities needed for the work. This facilitates communication and streamlines experimental workflows which saves time and costs. CoLabs also provides training related to the CoProject for the embedded researcher throughout the course of the collaboration.
Most or all experiments are performed in CoLabs facilities by some combination of CoLabs scientists and embedded researchers. Regular meetings are held to review progress and data, plan upcoming tasks, and change the scope as needed. Collaborators are also expected to participate in our CoLabs-wide seminar series and are invited to attend our yearly retreats.
FAQs
1. Can CoLabs help generate preliminary data for a grant application?
Yes! Our CoProjects range from smaller pilot studies to larger investigations. We work with you to design the project that best fits your goals and financial obligations. Please contact us to get started.
2. What is the difference between recharge and support for a CoProject?
Recharge is a fee-for-service mechanism for discrete instrument use, consultation, etc., typically with a single CoLabs Team. To get started, please see Flow and Mass Cytometry, Optical Microscopy, Genomics, and Spatial Omics.
Support for CoProjects is typically provided by a grant or contract. These funds provide salary support for CoLabs Team members and related costs of the CoProject done in CoLabs. We work with you to secure funding for your CoProject. Please contact us to learn more.
3. Does my study qualify for a CoProject?
CoProjects typically combine expertise and equipment from multiple CoLabs Teams, require data integration and analysis from multiple sources, and involve close day-to-day collaboration with CoLabs scientists. These are large-scope, multi-modal research projects, though the sample size can range from pilot studies to full-scale investigations. They generally take one or more years to complete. Check out our Portfolio for current and previous CoProjects. If you have further questions or would like to get started, please contact us.
For projects and experiments that do not fit the CoProjects model, CoLabs offers many other ways to collaborate with us. Please see how you can work with us and feel free to reach out for more information.
4. Do I need to be in UCSF Parnassus to work with CoLabs? Do I need to be an ImmunoX member?
No, we collaborate with people across all campuses and affiliations, including external investigators.