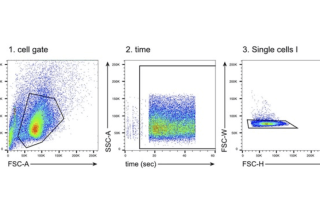
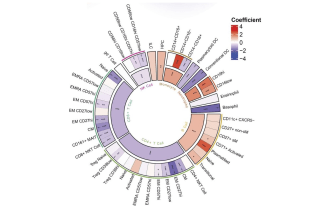
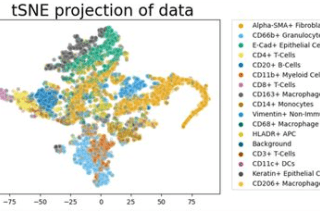
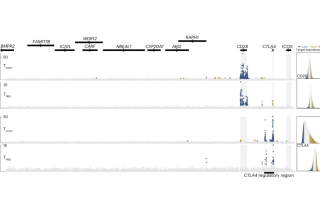
CoLabs supports UCSF and external investigators using flow and mass cytometry for cell analysis and sorting.
CoLabs maintains a large collection of instruments for independent use, provides scientists with training and education courses, and offer consultation. More information, including recharge rates, can be found on the Flow Cytometry CoLab website.
To get started, please contact Flow Cytometry CoLab. We can assist you with training requirements, review your experimental design, and discuss next steps. From there, you may work with additional CoLabs Teams depending on your project. Along with flow and mass cytometry, you can take advantage of the complementary platforms, expertise, and collaborative culture at CoLabs. Our labs are located near each other to streamline communication and workflows.
Working within the CoLabs Community
If you plan to sort for single cell genomic approaches, please contact Genomics CoLab to schedule a preliminary meeting. For assistance with sample preparation and panel design, please contact the Disease to Biology CoLab which has experience with many sample types. To combine cytometry data with other data, contact Data Science CoLab to discuss options for data integration and analysis. For large-scope projects, you can establish a multidisciplinary collaboration (“CoProject”) between you and multiple CoLabs Teams. CoProjects is also a conduit for CoLabs to develop customized methodologies.
Flow cytometry sorters are equipped with up to 7 lasers and can detect up to 60 parameters. These instruments can sort up to 6 different populations into tubes or plates or onto slides, including single cell sorting for downstream applications such as RNA-sequencing. We are one of the few facilities that can sort billions of cells for approaches such as CRISPR screening. In addition, we sort BSL-3 samples at Zuckerberg San Francisco General.
Conventional flow cytometry analyzers have up to 5 lasers and detect up to 30 parameters. Select instruments have the ability for high throughput sampling in 384 well plates. We also offer instruments capable of detecting 10 – 2000 nm nanoparticles, for applications such as drug development, protein aggregation, and exosome characterization.
Spectral flow cytometry analyzers capture each fluorochrome’s full emission spectrum to increase the number of parameters that can be measured. Our spectral instruments have 5 lasers with a total of 64 detection channels and an automatic micro-sampling system for 96-well plates.
CyTOF mass cytometers use elemental, instead of fluorescent, tags. Capable of detecting 48 parameters, this approach is useful if you have limited sample and want to identify markers of interest before shifting to more standard flow cytometry. It can also be used for direct comparison of expression levels between up to 20 samples in a single tube.
Detailed configurations for each instrument can be found here.
We also have multiple analysis workstations available for reservation. Licensed software includes FCS Express, FlowJo, and Cytobank.
We offer several levels of assistance for instrument use. New users are required to attend an onboarding session, offered weekly.
Once training is complete, many of our instruments are available for fully independent use. Guidance service is available as a refresher and/or for configuration review before users perform their own acquisition. For those who prefer a higher level of support, CoLabs can run your samples through our Operator Assistance service.
In-person educational courses on the principles of flow and mass cytometry, panel design, software introduction, and more are offered monthly. Many include a hands-on component at the instrument to run test samples.
Free, virtual consultations are available upon request. We are happy to share our standard panels, recommend verified methods for sample processing, and help you customize protocols for your experiment. Office hours are generally on Mondays at 1-4 pm.
We have collected recommended protocols and guidelines and other references on our instruments to help you get started.
One of the goals of CoLabs is to promote standardized protocols and data sharing. Our Flow Cytometry, Disease to Biology and Data Science CoLabs have developed new methods for sample processing, panels, and analysis tools for cytometry experiments.
In our Cyclone analysis pipeline, we integrated multiparametric data from mass cytometry, spectral flow cytometry, and multiplexed immunofluorescence. This pipeline can be used in a variety of biological contexts. See the Cyclone package and documentation to determine if your data is suitable. You can also contact the Data Science CoLab to customize programs for analysis.
In addition, many flow and mass cytometry datasets are available at the UCSF Data Library, which is curated and maintained by our Data Science CoLab. These datasets are available for researchers to explore and analyze.
1. Where are flow and mass cytometers located?
Most of our instruments are located at UCSF Parnassus Heights on the 8th floor of the Medical Sciences Building. We share the floor with other CoLabs teams to facilitate experimental workflows and communication.
We also support instruments at the BSL-3 facility in Pride Hall at Zuckerberg San Francisco General Hospital.
2. What if I don’t see an instrument that can do what I need for my research? Can CoLabs manage flow cytometers purchased by UCSF labs or programs?
The Flow CoLab is enthusiastic about partnering with UCSF investigators and programs to obtain and manage additional cytometers that augment our capacity and bring novel capabilities to UCSF research community.
3. Who do I contact for more information on training, questions about instrument capabilities, help with experimental design, etc?
flowassist@ucsf.edu
Medical Sciences Building S854
513 Parnassus Ave (map)Additional information can be found at the Flow Cytometry CoLab website.